What is Preimplantation Genetic Testing for Aneuploidy?
AboutPreimplantation genetic diagnosis is a test to check for chromosomal abnormalities using cells sampled from blastocysts created through microinsemination. Also possible through this method is Preimplantation Genetic Testing for Aneuploidy (or PGS; Preimplantation Genetic Screening) or PGT-A.
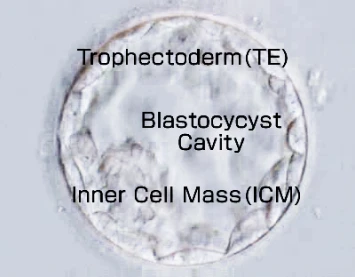
Around 5~7 days after fertilization a sample portion of trophectoderm cells (the cells that will form the placenta) are taken from the blastocyst, DNA is extracted, amplified and then analyzed by a method called Next Generation Sequencing (NGS).
PGT-A (PGS) Using Cells and niPGT-A (niPGS) Using Culture Medium
There are two methods of PGT-A (PGS).
- A method using the part that will become the placenta (TE cells)
- A method using the embryo culture medium
One method is the conventional PGT-A (PGS) that uses TE cells.
A portion of the TE cells (the part that will become the placenta) is collected from a blastocyst 5–7 days after fertilization. DNA is then extracted and amplified from these cells and analyzed using NGS.
The blastocyst from which TE cells are collected is cryopreserved until the test results become available.
The other method is niPGT-A (niPGS), which uses the culture medium. Because TE cells are not collected, this is a newer testing method that is gentler on the embryo.
The culture medium in which the embryo has been grown to the blastocyst stage on day 6 or day 7 after fertilization is collected, and DNA is extracted and amplified from it for analysis using NGS.
As with conventional PGT-A (PGS), the blastocyst is cryopreserved until the test results are available.
niPGT-A (niPGS) is an abbreviation for non-invasive PGT-A (non-invasive PGS), meaning a form of PGT-A (PGS) that does not damage the embryo (non-invasive).
Benefits and Risks
Benefits & RisksBenefits
It is known that many cases of implantation failure and miscarriage are caused by chromosomal abnormalities in the fertilized egg (embryo).
If the presence or absence of chromosomal abnormalities in embryos intended for transfer is known in advance, embryos that are unlikely to result in pregnancy can be excluded from transfer. This can reduce the miscarriage rate and eliminate unnecessary time spent on infertility treatment.
In addition, if a serious congenital disorder is detected through prenatal testing during pregnancy, patients may be faced with the difficult decision of whether to continue the pregnancy. By performing PGT-A, the likelihood of congenital disorders caused by chromosomal abnormalities can be reduced.
Risks
Because it takes several weeks for the analysis results to become available, fresh embryo transfer cannot be performed when PGT-A (PGS) is used.
The blastocyst from which the sample is collected is cryopreserved, and a frozen-thawed embryo transfer is performed based on the PGT-A (PGS) results.
| niPGT-A (niPGS) | Conventional PGT-A (PGS) | |
|---|---|---|
| Benefits |
|
|
| Risks |
|
|
*Horizontal scrolling is available
[Important Notice]
Even if an embryo in which no chromosomal abnormalities were detected by PGT-A (PGS) is transferred and results in pregnancy and delivery, it is rare but possible for the child to be born with a chromosomal abnormality.
Possible reasons include microdeletions that cannot be detected by NGS, or cases in which the chromosomes of the TE cells (the part that will become the placenta) used for testing differ from those of the cells that will become the fetus (ICM), known as mosaicism.
In addition, with PGT-A (PGS) using TE cells, there is a possibility of damage to the blastocyst, such as fragmentation or developmental arrest, due to the removal of TE cells (PGT-A itself does not induce chromosomal abnormalities).
The results of niPGT-A (niPGS) and conventional PGT-A (PGS) may not always be consistent.
This is because, in the conventional method, only a portion of the embryo is collected and tested; therefore, in the case of a mosaic embryo, other areas may yield results different from those of the tested portion.
Conventional PGT-A (PGS) and niPGT-A (niPGS) cannot be used simultaneously on the same embryo, nor can both tests be used concurrently from the same egg retrieval cycle.
Methods
Methods⑴ Egg retrieval, intracytoplasmic sperm injection (ICSI), and blastocyst culture are performed.
⑵ TE biopsy is performed (this involves collecting TE cells from the blastocyst).
⑶ Chromosomes are amplified using the PCR method.
⑷ Chromosomes are analyzed using a method called next-generation sequencing (Next Generation Sequencing; NGS).

About Results
Test Results With NGS, it is possible to determine how many copies there are of each autosome from chromosome 1 to chromosome 22, as well as the sex chromosomes (X chromosome and Y chromosome).
The NGS analysis results are displayed in graph form as shown below. When a chromosome has three copies (trisomy), it is plotted on the blue line; when a chromosome has only one copy (monosomy), it is plotted on the red line.
Normally, autosomes are present in two copies each, so if there are no chromosomal abnormalities, they are plotted in a straight line along the baseline.
For sex chromosomes, females have two X chromosomes, so they are plotted on the baseline; because there is no Y chromosome, it is plotted below the monosomy line.
In males, there is one X chromosome and one Y chromosome, so they are plotted on the monosomy line.
*Please enlarge the image to confirm.
[Graph 1] No chromosomal abnormalities, male (XY)
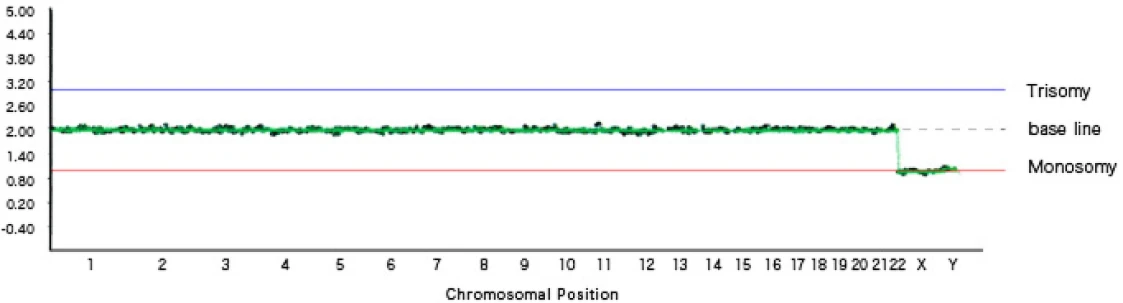
In Graph 1, chromosomes 1 through 22 are plotted on the baseline, and the X chromosome and Y chromosome are each plotted on the monosomy line, indicating a male with a normal number of chromosomes.
[Graph 2] Trisomy 9, monosomy 18, sex chromosome abnormality (XXY)
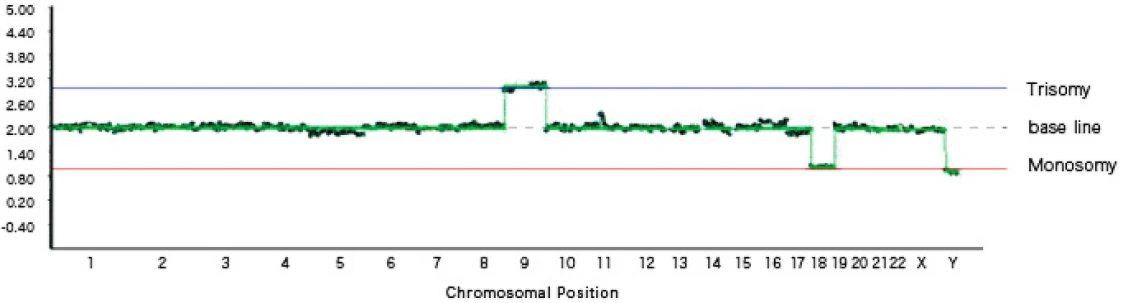
In Graph 2, trisomy of chromosome 9 and monosomy of chromosome 18 are observed. In addition, a sex chromosome abnormality is present, with a total of three sex chromosomes (two X chromosomes and one Y chromosome).
[Graph 3] Mosaic trisomy 3, mosaic monosomy 16, female (XX)
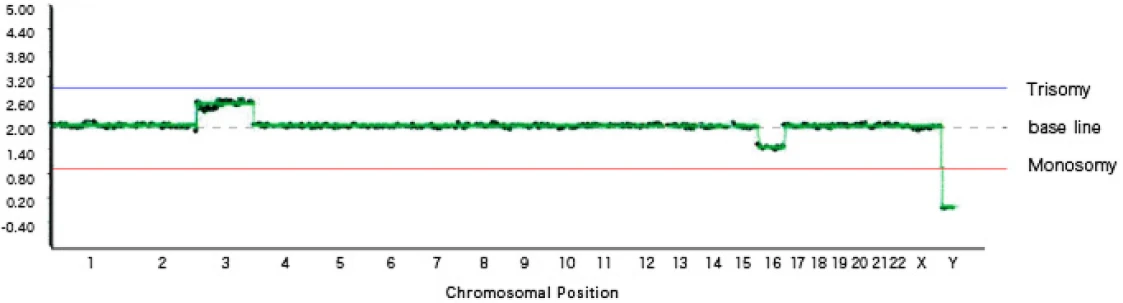
Graph 3 shows mosaicism.
Mosaicism refers to a condition in which cells with chromosomal abnormalities and cells without chromosomal abnormalities coexist within a single blastocyst.
On the graph, mosaic trisomy is plotted between the trisomy line and the baseline, while mosaic monosomy is plotted between the monosomy line and the baseline.
In this graph, mosaicism is observed in two locations: chromosome 3 shows a mixture of normal and trisomic cells, and chromosome 18 shows a mixture of normal and monosomic cells.
*There is no official consensus among specialists regarding the management of mosaic blastocysts; however, it is known that, depending on the degree of mosaicism, a certain proportion of healthy children are born.
How to Apply
How to ApplyFirst-Time Applicants
After a telephone consultation, we will refer you to a designated medical institution.Please first contact us at english_help@ogms.biz.
Returning Applicants
After reading “Explanation of Preimplantation Screening (PGT-A, PGS)”, please send the information listed below to english_help@ogms.biz.
In addition, please submit the “Consent Form for Preimplantation Screening (PGT-A, PGS)” by mail.
The mailing address for the consent form will be provided after we receive your application email.Your application will be considered complete upon receipt of both the application email and the original consent form.
Information to Include in the Application Email
Subject: PGT-A Application
Body:
① Full name
② Address
③ Phone number
④ Name of the medical institution where egg retrieval will be performed and patient ID number (Note: this is not a shared ID within a medical corporation, but an individual ID specific to each clinic.)
*If you are receiving care at a medical institution other than one designated by our company, we will provide separate guidance. Please contact us first.
Emails received at night, on weekends and public holidays, during the year-end and New Year holidays, or during summer holidays may receive delayed responses. In addition, depending on the content of your inquiry, it may take some time for us to respond.

